U.S. Army General Gustave Perna has called today D-Day, a reference to the Allied landing in France during WWII — the beginning of the end in a historic battle against an enemy that has taken hundreds of thousands of lives and threatened basic freedoms.
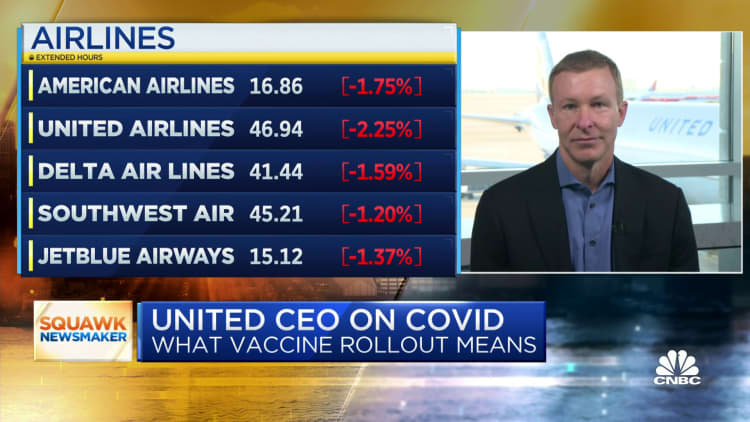
For UPS, today is the "moment of truth" after months of logistical planning.
The United States' government, with the help of UPS and FedEx, is now moving rapidly to deliver millions of doses of Pfizer's coronavirus vaccine, an accomplishment that President Donald Trump praised as a "medical miracle." House Speaker Nancy Pelosi is now calling for the invocation of the Defense Production Act to accelerate manufacturing of the vaccine.
It's now a race against time as thousands of people die every day, alone and separated from their families, from a virus that has devastated communities in every corner of the nation and shaken America's way of life to its core.
Though help is on the way, the situation is expected to get much worse before it gets better. At least 3,300 deaths from the virus were recorded on Friday and more than 231,000 new cases were reported, stretching the U.S. health-care system to the brink.
With a hard winter ahead, CDC advisors are sounding alarm bells that there's still not enough federal funding to help local authorities carry out the vaccination campaign. Congress remains at an impasse over a new stimulus package that would include such funding.

The following data was compiled by Johns Hopkins University:
- Global cases: More than 71.2 million
- Global deaths: More than 1.5 million
- U.S. cases: More than 15.8 million
- U.S. deaths: More than 295,000
Here's what you need to know:
- D-Day: Army general says vaccine beginning of end for virus
- First vaccine shipments arrive Monday at U.S. locations
- For UPS, the "moment of truth"
- FDA chief: Vaccine authorization not due to external pressure
- Patients must receive two shots for full protection: FDA
- Here's who will get the vaccine first
- Allergies: Injection okay unless you've had reaction to a vaccine
- Trump praises vaccine as "medical miracle"
- Pelosi: Time to invoke Defense Production Act