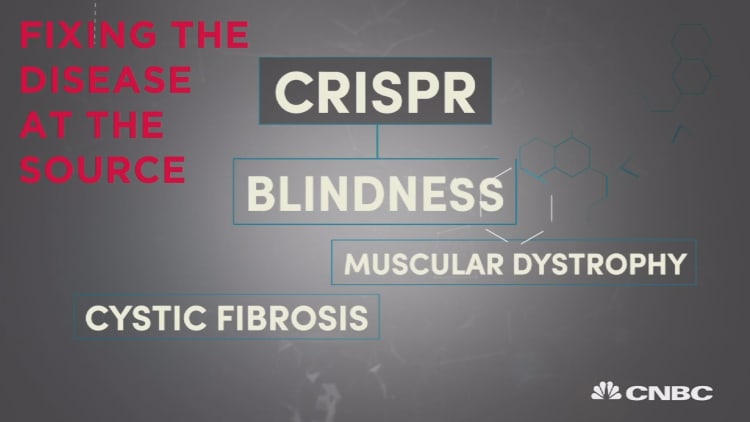
The recently discovered, hotly contested gene-editing enzyme CRISPR is poised to change the way we treat, even cure, many diseases. But treatment will come years before talk of any "cure" is reasonable, and researchers know where they'd put their money if they had to bet on which kind of disease will be the first to the clinic. Bet on CRISPR succeeding first against a rare, single mutation.
Researchers at a growing number of companies and academic institutions have already launched nearly a dozen efforts to craft novel treatments for conditions that have a genetic component, such as Duchenne Muscular Dystrophy, lung cancer, metabolic liver disease, congenital blindness, blood disorders like hemophilia and sickle cell disease, Huntington's disease and cystic fibrosis.
Mark Kay, a professor of pediatrics and genetics at Stanford University, is placing his bet on a treatment for rare immunodeficiency conditions, such as the "bubble boy" disease, which he says could come about in the next five years or even sooner. These diseases top researchers' lists because they are usually caused by a single, clearly defined mutation, giving researchers an obvious target to cut out or replace with functional DNA.
But anticipating when CRISPR could be used to develop a cure is more difficult to assess. There are not only technical hurdles to overcome but ethical ones. An influential U.S. science advisory board recently, and for the first time, gave cautious approval for gene editing in embryos to prevent diseases such as Huntington's and Tay-Sachs, but not for noncritical research efforts, such as the so-called designer babies or even for diseases for which there is an alternate treatment.
"When people talk about a cure for whatever disease [with CRISPR], that's a tough one," said David Edgell, an associate professor of biochemistry at the University of Western Ontario.
Scientists are first looking to use gene therapy tools on a certain number of cells to treat a disease, a more attainable goal. Some treatments, such as those for cancer involving modified T-cells and blood diseases with stem cells in bone marrow, allow scientists to remove pertinent cells from the body, modify them with CRISPR, and put them back in. Others, such as eye diseases or cystic fibrosis, have to be altered in the living patient's cells. Additional factors, like how many cells have to be affected for the symptoms to subside and how easily researchers can access the pertinent cells — such as whether they are in the eye or deep in the bone marrow — can also influence the techniques researchers use to treat disease.
But all that assumes CRISPR works flawlessly. Scientists still have some questions. "We know CRISPR works and that it works well. But there are challenges in terms of getting therapy to work in humans, to ensure its efficiency, delivery and safety," said Charles Gersbach, an associate professor of biomedical engineering at Duke University.
There's some evidence that CRISPR might make some unintended changes to other parts of the genome, called off-target effects. No one is quite sure how often this happens. If scientists wanted to use CRISPR to cut one mutation out of a patient's DNA and replace it with functional DNA, there's no foolproof way to make sure that CRISPR made only the one cut instead of 10 unintended edits, called off-target effects.
"People have done work to minimize off-target effects. There's been progress, but it's not zero and it never will be," Stanford's Kay said.
Limiting the amount of time CRISPR can work in cells could reduce the likelihood that erroneous cuts are made. Researchers are developing a CRISPR "off-switch," a protein they can inject into affected cells that, when deployed, would make CRISPR stop cutting to reduce off-target effects.
Delivering CRISPR into a living patient comes with additional challenges, since the enzymes in many cases have to be delivered with viruses. Though many of these viruses are thought to be harmless and won't infect patients, some studies suggest that they could cause an immune response in the patient that could be damaging. Scientists are discovering new workarounds, however. A recent experiment to treat muscular dystrophy in mice skipped the viruses and injected the gene-editing proteins directly into the cells' DNA.
More from Modern Medicine:
Treating rare diseases by robot
Edited DNA is OK for some things, but not designer babies
Chinese first to test CRISPR in humans
Once scientists are confident that a CRISPR-based treatment can be used safely and effectively in the lab, they have to test it on large animals such as cows and pigs, then on humans in clinical trials. These experiments often take years, in part because the researchers want to see how long the treatment lasts and to evaluate any unintended long-term consequences, such as increased incidence of cancer. The Food and Drug Administration, which approves new treatments, has stated that it will evaluate products, not technology. Gersbach of Duke said that will mean each individual approval will take longer than if there was a designated pathway for CRISPR-based treatments. Licensing CRISPR for clinical use might also be a hurdle in light of the recent legal battle over who owns the patent for it. Edgell said it might already be slowing innovation.
In spite of these hurdles, researchers don't doubt that CRISPR-based treatments will become widely available to patients. The only question is when. CRISPR isn't the first enzyme complex poised to alter genes to treat or cure disease, joining other proteins such as TALENS and zinc-finger nucleases. Gene therapy treatments using zinc-finger nucleases and TALENS are likely to beat CRISPR to the clinic, as trials are under way to treat diseases such as hemophilia, leukemia and HIV. But CRISPR is faster and easier to use than its predecessors, giving scientists the ability to target specific areas of the genome to cut up and edit, Kay said.
CRISPR won't be far behind. Edgell thinks CRISPR treatments could be available within the next two to three years, with modified T-cells used to treat some types of cancer (there are already clinical trials for lung cancer in China, and a similar one slated to take place at the University of Pennsylvania was approved last June by the National Institutes of Health).
Progress, Kay warns, may not be linear. There will be some treatments that work and some that don't. "There's no question that [CRISPR] is having a huge effect on developing new therapies to treat human disease," Gersbach says.
He's excited about it but is "also aware of the challenges of translating those things in a rigorous and safe way from mice to humans. No one should expect it to happen overnight. But everyone does expect that it will eventually have an effect on particular diseases."
— By Alexandra Ossola, special to CNBC.com