Vertex Pharmaceuticals' stock soared Tuesday after studies showed that a new combination of drugs from the company met the goals of two late-stage clinical trials in cystic fibrosis, setting the stage for Vertex to apply for regulatory approval in the fourth quarter.
At one point, the stock was trading at its highest level since October 2000. (Click here for the latest price.)
The medicines, Kalydeco and lumacaftor, improved lung function by an average of 2.6 to 4 percentage points from patients' baseline levels versus placebo, Vertex said in a statement Tuesday. The studies, dubbed Traffic and Transport, were from the third and final phase generally required for approval.
The results were among the most anticipated events in the biotechnology industry this year, analysts said. They projected Vertex's stock, which closed at $66.61 Monday, could rise above $100 on positive results, or sink to the $40s on negative results. They also said the data could have broader industry implications.
Read MoreThe next UK pharma takeover target may be…
"Given that Vertex is one of the largest companies in the space, the results of Traffic/Transport will have a significant effect on sentiment for biotech," Ravi Mehrotra, an analyst with Credit Suisse, wrote in a research note before the data were released.
In 2012, Vertex received approval for Kalydeco—the first medicine to address the genetic cause of cystic fibrosis, a rare, inherited disease that causes buildup of sticky mucus in the airways and other areas, and is often fatal by age 40. Cystic fibrosis affects about 70,000 people worldwide, according to the Cystic Fibrosis Foundation, which contributed to the development of Vertex's cystic fibrosis drugs.
Read MoreInvestors eyeAchillion for next big pharma deal
While Kalydeco was a breakthrough for cystic fibrosis patients, it only addresses mutations possessed by a fraction of the population. Vertex is testing it in combination with lumacaftor, still an experimental medicine, to address more patients; study results published Tuesday were in a patient population that accounts for about 40 percent of the total population, Mehrotra said.
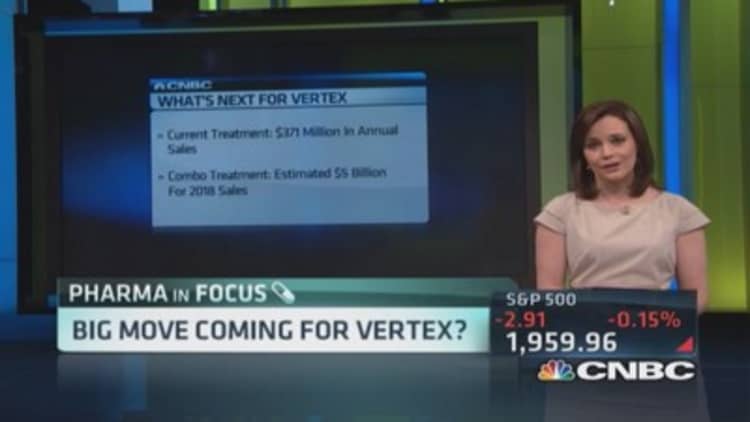
Kalydeco drew $371 million in 2013 revenue. In total, Vertex's cystic fibrosis medicines may draw more than $5 billion in annual revenue by 2018,according to estimates earlier this year from Phil Nadeau, an analyst with Cowen & Co.

The studies, which ran for 24 weeks, also met secondary goals, including reducing pulmonary exacerbations—or signs of the disease worsening and requiring treatment with antibiotics—and helping patients gain weight, Vertex said. The trials were conducted in more than 1,100 patients ages 12 and older with two copies of a mutation known as F508del.
"On average, people with cystic fibrosis who have two copies of the F508del mutation lose nearly 2 percent of their lung function each year, underscoring the urgent need for new medicines that address the underlying cause of this disease," Dr. Bonnie Ramsey, professor of pediatrics at the University of Washington School of Medicine and a lead investigator on one of the trials, said in Vertex's statement.
Read MoreWhat new GSK cancer deal shows about pharma market
The improvements in pulmonary exacerbations were especially notable, "given the potential for these events to result in hospitalizations, permanent lung damage, and the need for additional treatment with antibiotics and other medicines," she said.
The combination regimens were generally well tolerated, Vertex said. Slightly more than 4 percent of patients on the combination stopped treatment because of side effects, compared with 1.6 percent of those taking placebo.
More than 1,000 patients opted to take the combination regimen after the study ended, Vertex said, underscoring the need for medical options that analysts also noted leading up to the data's release.
Read MoreAllerganadvises shareholders against Valeant tender offer
"We can debate what difference each endpoints make on reimbursement/price/uptake, etc.," RBC Capital Markets' Michael Yee wrote in a June 17 research note, "but based on our recent discussion with key opinion leaders, as long as primary hits and the drug is approved, they intend to put all patients on drug."
—By CNBC's Meg Tirrell

