An experimental drug from Biogen and Japanese partner Eisai was shown to slow the declines in memory and thinking clearly associated with Alzheimer’s disease in a clinical trial, giving support to the thesis that targeting buildups of amyloid plaques in the brain can alter the course of the disease.
The drug, dubbed BAN2401, slowed declines associated with Alzheimer’s disease by 30 percent at its highest dose in the trial, compared with placebo, after 18 months, Eisai reported today at the Alzheimer’s Association International Conference in Chicago. The slowing in progression came as the drug dramatically reduced buildups of amyloid plaque in the brain, by 93 percent at the highest dose.
Investors had pegged success at a 20 percent slowing of Alzheimer’s progression or more at 12 months, rather than 18, making the results difficult to compare directly to expectations.
Biogen’s stock declined in after-hours trading, a move Piper Jaffray analyst Chris Raymond attributed to one measure of cognition – known as the Clinical Dementia Rating – that didn’t show a statistically significant improvement.
Lynn Kramer, chief medical officer of Eisai's neurology business group, declared success, noting Wednesday in a media briefing, “We think this result is really the first of its kind,” and “robust enough to approach regulatory authorities to discuss next steps.”
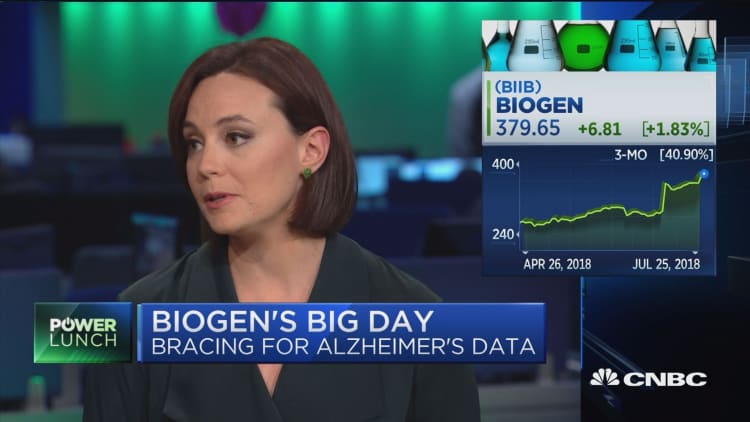
The companies surprised investors earlier this month when they announced the study had met its goal after 18 months, because it hadn’t done so at a 12-month look. Both Biogen and Eisai’s stocks rallied so much the companies collectively gained $20 billion in market value, according to Robert W. Baird analyst Brian Skorney.
The results were even more surprising given the poor track record in Alzheimer’s drug development, including trials of medicines that target amyloid plaques. At least nine major late-stage clinical trials of Alzheimer’s medicines (not all targeting amyloid) have failed in the last decade, though the amyloid hypothesis – which suggests targeting plaques will alter the disease’s progression – remains a prevailing theory in the field.
“This is the second trial that is demonstrating a clearance of amyloid in the brain through PET imaging, and perhaps other biomarkers,” Maria Carrillo, chief scientific officer of the Alzheimer’s Association, told reporters Wednesday. “And also has hints of some efficacy in cognitive tests.”
The first, she noted, was an earlier-stage trial of another Biogen Alzheimer’s drug, aducanumab, now in more advanced clinical trials expected to produce data in 2020.
“What we see is the amyloid hypothesis remains something that needs to continue to be tested,” Carrillo said.
The 30 percent slowing of Alzheimer’s progression in the results released Wednesday was measured by a scale called the Alzheimer’s Disease Composite Score, or ADCOMS, a combination of commonly used endpoints that gauge cognition, or the ability to remember things and think clearly.
On a more common measure of cognition, known as ADAS-cog, the highest dose of BAN2401 slowed progression by 47 percent compared with placebo. On another well-known measure, known as the Clinical Dementia Rating, or CDR, the difference between the highest dose and placebo was 26 percent, but that result didn’t meet statistical significance. That metric is particularly important because it’s the primary goal of Biogen’s ongoing aducanumab trial, Raymond wrote in a note to investors.
“We do think this reaction is misplaced and shares will ultimately recover once investors put these data into perspective,” Raymond wrote.
The most common side effects were infusion reaction and one known as ARIA, or amyloid-related imaging abnormalities, which can manifest as brain swelling or bleeding. Such swelling occurred in less than 10 percent of patients across all treatment groups, Kramer said.
The study, of 856 patients, was conducted in those with early Alzheimer’s disease, those with mild cognitive impairment due to Alzheimer’s, or mild Alzheimer’s dementia. More than 5 million Americans are currently estimated to have Alzheimer’s, a number that’s expected to nearly triple by 2050 without effective interventions.

