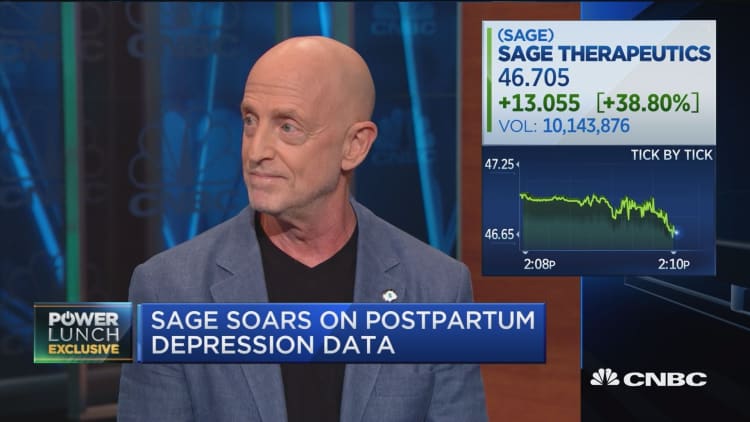
Shares of Sage Therapeutics skyrocketed Tuesday after the drugmaker announced positive results for a phase 2 trial involving a new drug to treat postpartum depression.
The stock closed up 37 percent. It had been up as much as 44 percent.
Postpartum depression is a mood disorder that can affect 1 in 7 women after childbirth. The company said data on 21 patients showed that the company's drug, SAGE-547, achieved a statistically significant reduction in symptoms at 60 hours, compared with a placebo, on a standard depression scale used in clinical research. The difference in treatment began at 24 hours and maintained through to the 30-day follow-up.
Similarly, at 30 days, seven patients in the SAGE-547 group and two in the placebo group were in remission.
Mothers with PPD can experience symptoms such as sadness, anxiety and exhaustion, according to the National Institute of Mental Health. There are no approved therapies specifically for PPD. Existing options include talk therapy and antidepressants.
"This is potentially one of the most important clinical findings in the pharmacologic treatment of postpartum depression to date," trial investigator Samantha Meltzer-Brody said in a press release.
Trial participants were required to have a HAM-D score, a rating scale used to provide an assessment of depression, of 26 or above. Remission was determined if the score reached 7.
Patients in the trial were required to have had a major depressive episode that began no earlier than the third trimester and no later than the first four weeks following delivery. They also must enroll within six months of having their baby.
The study adjusted dosage based on body weight. And all patients tolerated the drug fairly well, according to the report.
The company has initiated an expansion of this phase 2 clinical program to determine optimal dosing of SAGE-547 in PPD with enrollment to begin by the end of next year, according to the report. In addition, the company seeks regulator input "to determine the appropriate pathways for developing both medications for this indication."
The company is about to begin a larger study looking at women with moderate and severe forms of postpartum depression, Jeff Jonas, CEO of Sage, told CNBC's "Power Lunch" Tuesday.
"We believe these women have an acute deficiency. A brain hormone is lowered normally around birth and some women appear to be vulnerable to the imbalance that causes. Our drugs target that imbalance and we believe regularize or normalize brain function," he said.
Sage shares have plummeted this year, falling more than 20 percent.
SAGE 2016 Chart


