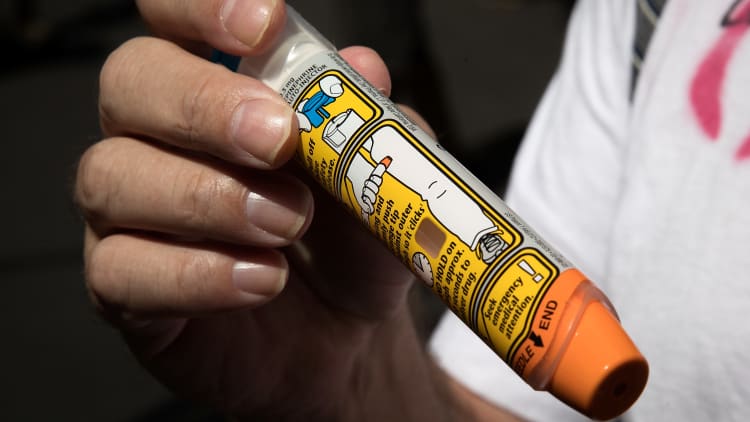
American taxpayers may have been overcharged for EpiPen anti-allergy devices by as much as $1.27 billion over the course of a decade, a U.S. senator said Wednesday.
That amount is nearly three times the $465 million that EpiPen's owner, the big drugmaker Mylan, last October said it agreed to pay the federal government to settle claims that it overcharged the government-run Medicaid system for the devices.
"The fact that the EpiPen overpayment is so much more than anyone discussed publicly should worry every taxpayer," said Sen. Chuck Grassley, R-Iowa, chairman of the Senate Judiciary Committee.
Grassley said he learned of the large disparity between Mylan's settlement amount and the potential overpayment by taxpayers from the Department of Health and Human Services' Office of Inspector General after asking officials for an accounting of EpiPen overcharges.

Mylan for years classified EpiPen as a generic drug for the purposes of Medicaid's drug rebate program, and as a result paid a lower rebate rate to Medicaid than did sellers of brand-name drugs. Officials have said that EpiPen, which is used to counteract a potentially fatal allergic reaction known as anaphylaxis, should have been treated as a brand-name product for Medicaid's rebate program.
Medicaid is the joint federal-state program that provides health-care coverage to primarily low-income Americans.
Grassley also said the federal Centers for Medicare and Medicaid Services "recently provided records to the committee that show Mylan was made aware of the misclassification years ago but did nothing."
"It looks like Mylan overcharged the taxpayers for years with the knowledge EpiPen was misclassified, and the previous administration was willing to let the company off the hook," Grassley said, referring to the Obama administration.
A spokeswoman for Mylan said, ''We have no comment beyond that we continue to work with the government to finalize the settlement as soon as possible.''
Mylan faced strong public criticism last year for hiking the price of EpiPen to more than $600 for a two-pack, an increase of more than 500 percent in recent years.
Outrage over the cost led Mylan to increase the amount of financial assistance it gave customers, and to later introduce a generic version of EpiPen that sold for half of the price of the brand-name version.

Amid that controversy, a number of elected officials questioned whether Medicaid had been receiving the correct amount of rebates from Mylan for EpiPen sales within that program.
Mylan for weeks had repeatedly denied underpaying the rebates. But, in October, it agreed to a settlement with the Justice Department to resolve such claims. It also agreed to treat EpiPen as a brand-name product for the purpose of rebates beginning this past April.
However, in March, Mylan refused to say whether it would begin paying the higher rebate rate in April as scheduled.
The rebate program imposes a 13 percent rebate on generic drugs. But sellers of brand-name drugs are required to pay a rate of at least 23.1 percent.
However, the rebate for brand-name medications can be much higher if their sellers have raised the price of the product beyond the rate of inflation, as Mylan repeatedly did with EpiPen. In fact, Mylan's rebate rate for EpiPen could be so high that the company might have to pay Medicaid back nearly all of the money it made from selling the devices through the program.
Mylan was sued in April by customers who claimed the company engaged in an illegal scheme to dramatically increase the price of EpiPen over the past decade.
The suit alleged that the "skyrocketing" list price of EpiPen was the result of Mylan's payments of rebates to pharmacy benefit managers — including CVS Caremark, Express Scripts and Optum Rx — which handle prescription drug benefit programs for insurance plans.
Mylan did not comment on the suit when it was filed.
Watch: Mylan CEO says system is broken