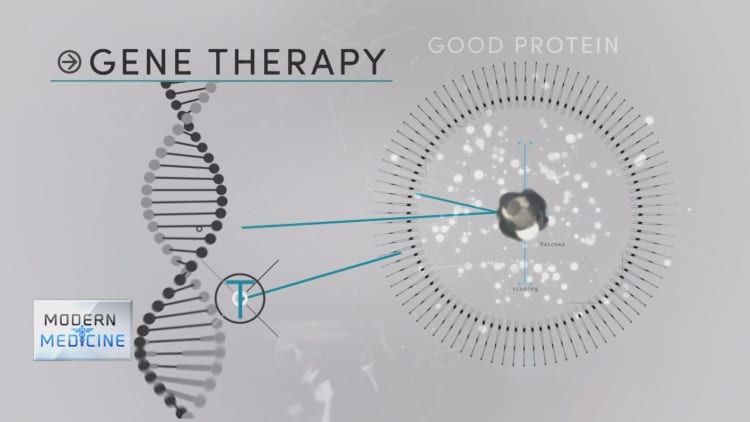
The Food and Drug Administration has approved Spark Therapeutics' Luxturna, the first directly administered gene therapy approved in the U.S. that targets a disease caused by mutations in a specific gene.
The treatment would help those with Leber congenital amaurosis, a rare genetic retinal disease. It's specifically approved for people who have a mutated RPE65 gene, which is vital to creating an enzyme needed for normal vision. A faulty one can make the gene less active or inactive, leading to impaired vision and even complete blindness.
Luxturna uses a modified virus to deliver a healthy copy of the gene directly to a patient's retinal cells through eye surgery. The working copy then replaces the broken one to spark production of the necessary enzymes to restore the patient's vision.
"Today's approval marks another first in the field of gene therapy — both in how the therapy works and in expanding the use of gene therapy beyond the treatment of cancer to the treatment of vision loss — and this milestone reinforces the potential of this breakthrough approach in treating a wide-range of challenging diseases," FDA Commissioner Scott Gottlieb said in a statement.
Luxturna is the third gene therapy approved by the FDA.
The agency's approval of Luxturna was widely expected. Spark Therapeutics shares rose as much as 6.8 percent before turning negative and closing down 0.2 percent on the day.