Sarepta Therapeutics CEO Douglas Ingram told CNBC that more tests are needed to develop a gene therapy to treat a rare genetic disease.
On Tuesday, the medical research and drug development company released data on an experimental treatment for patients with Duchenne muscular dystrophy, or DMD.
"This is preliminary results," Ingram said on "Closing Bell" on Tuesday. "We need to be careful."
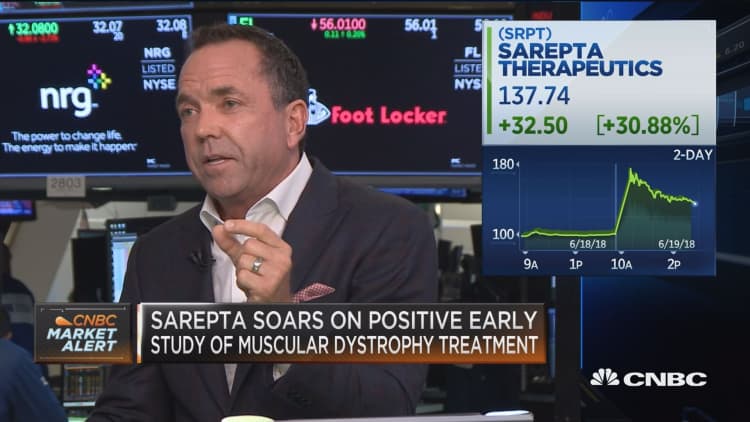
The successful preliminary trial sent shares of Sarepta Therapeutics soaring and opened the door for further testing.
The initial tests, on just three children during a three-month period, are "potentially transformative," Ingram said, but he acknowledged that more research needs to be done.
"We need to treat more kids," he said. "We're going to treat 12 kids versus 12 placebo kids. And we need to watch them for some time, perhaps a year."
"But we don't want to wait that long," he said. "Because every single day that we delay, these kids are being damaged. People have been trying to find a solution to this for decades."
A lack of dystrophin, a protein that helps keep muscle cells intact, causes the disorder and results in muscle degeneration and weakness. It affects boys predominantly. About 16 out of every 100,000 people have the disease, according to the Centers for Disease Control and Prevention.
People with the disease usually require a wheelchair by around age 11 and have a life expectancy of 20 to 25 years old, Ingram said.
"These kids have muscle damage every time they move," he said.
In damaged muscles the enzyme creatine kinase can leak into the bloodstream. Large amounts of the enzyme can signal Duchenne muscular dystrophy.
In the preliminary tests, patients were given a gene therapy that acted as a substitute for the dystrophin.
The results of the trial showed a nearly 90 percent average reduction of creatine kinase levels after using the company's drug treatment.
The company's shares surged more than 50 percent Tuesday after the results of the trial were announced. Sarepta Therapeutics' stock closed up more than 36 percent Tuesday.
Ingram did not comment on the potential price of the drug. He said if results of larger tests are also positive, then the company can seek FDA approval.
"Our goal, eventually, is to get this therapy to all patients," Ingram said. "That means we have to finish the clinical trial."
— CNBC's Angelica LaVito contributed to this report.
Correction: This story has been corrected to reflect that Sarepta’s Duchenne muscular dystrophy program was never on clinical hold at the FDA.


