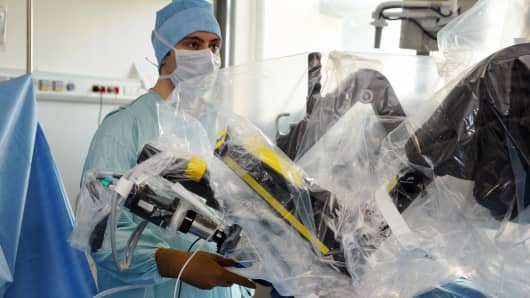
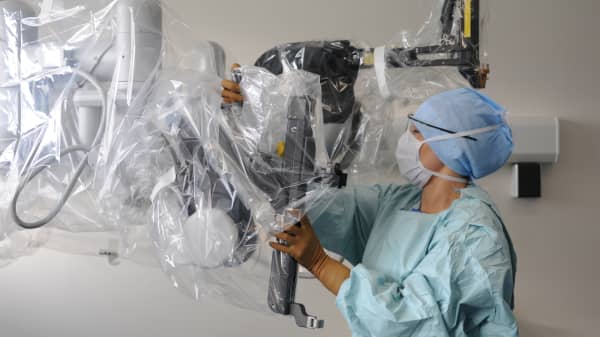
The Food and Drug Administration says Intuitive Surgical, which makes the da Vinci surgical robot, broke procedures when it warned customers about problems with the robot without first alerting regulators.
In what is known as a "483" letter following a recent inspection the FDA said that between January 2010 and December 2011 Intuitive received 134 complaints and filed 83 medical device reports related to "tip cover issues."