Europe's major pharmaceutical companies are under pressure to increase returns after a series of high-profile setbacks that have hit investors' and the general public's confidence.
Shares in Novartis, Europe's third-largest pharma firm by sales, have been pressured by a slew of disappointing news in recent months and were down nearly 6% so far this year before Tuesday's session, more than the European sector.
"Novartis Q2 results were overall slightly ahead of our forecasts and consensus, thanks to better than expected organic growth as well as lower than expected tax payments," WestLB
analyst Andreas Theisen said in a note.
Net profit at Novartis from continuing operations rose 18% to $1.94 billion in the second quarter, in line with analysts' forecasts, on the back of a 10% rise in sales.
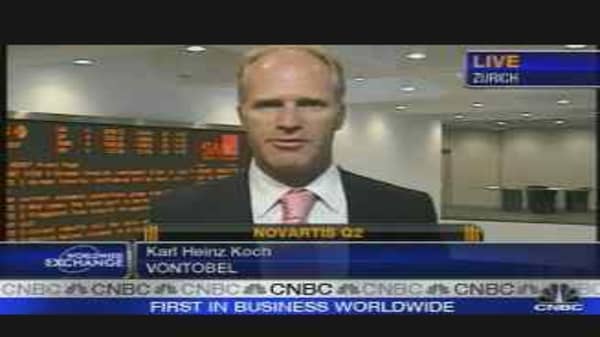
Although earnings guidance has been lowered, this had been flagged by the company and was probably priced into shares, said Vontobel analyst Karl-Heinz Koch.
Novartis also said U.S. regulators had extended their review period for cancer drug Tasigna by three months to review additional data. No new studies are required.
"The delay in the Tasigna approval will not change our estimates significantly, but further increases the risk profile of Novartis," Kepler Equities analyst Denise Anderson said in a note.
The drugmaker sweetened the pill for shareholders by pledging to complete previously announced share buybacks and purchase the remaining open amount of up to $4 billion in shares by February 2008.
The company said it would use its free cash flow and proceeds from two divestments to Nestle to fund targeted acquisitions and share buybacks.
Slowing Growth
Flagship products Diovan for high blood pressure and Glivec for cancer helped boost sales in Novartis's pharmaceuticals division by 6% to $6.06 billion.