
Johnson & Johnson hopes to begin human clinical trials on a COVID-19 vaccine in early November, its chief scientific officer, Dr. Paul Stoffels, told CNBC on Tuesday.
"We are making significant progress and very, very fast," Stoffels said on "The Exchange."
Stoffel's comments come one day after the first early-stage human trials for a potential vaccine to prevent COVID-19 began in Seattle. The National Institutes of Health is working with biotech company Moderna on the vaccine development.
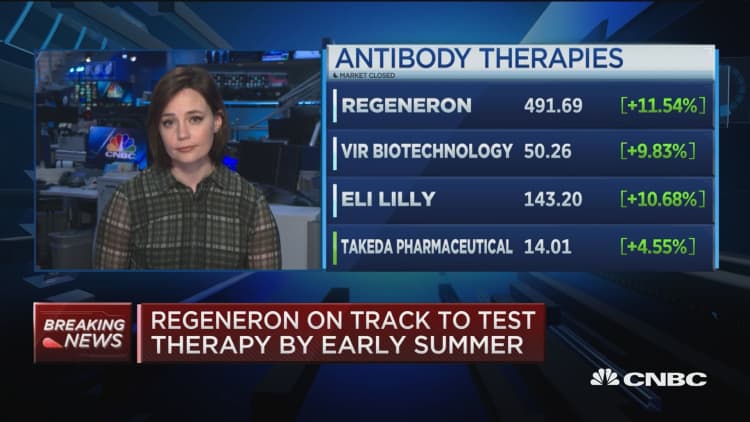
Regeneron on Tuesday said it aims to have a potential treatment for the coronavirus ready to start human clinical trials by early summer.
There is no known treatment for COVID-19, which has infected more than 190,000 globally and killed more than 7,500, according to Johns Hopkins University data.
Johnson & Johnson's work on a vaccine began in January. Stoffels said the drugmaker will probably have selected a candidate vaccine by the end of March, setting the stage for pre-clinical to begin.
At the same time, Johnson & Johnson will be putting processes in place to allow for the widespread production of the vaccine, "in order to have large quantities available early next year," Stoffels said.
Johnson & Johnson is using the same vaccine platform it most recently used for Ebola, Stoffels said. "So we can plug in the vaccine into an existing system and that allows us to go very fast with very good information," he said.
Johnson & Johnson's stock rose more than 7% Tuesday to $136.59.
Pfizer's vaccine efforts
Pfizer Chief Scientific Officer Dr. Mikael Dolsten told CNBC on Tuesday the company hopes to begin human trials on a potential COVID-19 vaccine in a few weeks.
That vaccine was being developed by German firm BioNTech, which announced a partnership with Pfizer on Tuesday to now co-develop it and distribute it outside of China.
"The timeline is very aggressive," Dolsten said on "Power Lunch." "We are just weeks away from starting dosing."
The companies had previously been working together on flu vaccine innovations for about two years, Dolsten said. He said Pfizer's history as a proven vaccine leader and BioNTech's expertise on messenger RNA, or mRNA, therapeutics make them strong collaborators on the coronavirus response.
"I think that supplements nicely what NIH and Moderna are doing and gives hopefully patients and all American people more options and more probability that we will come out successful," he said.
Shares of BioNTech hit an all-time high Tuesday following news of the Pfizer collaboration, rising 66.5% to $66.60 per share.
Pfizer's stock also was up on the news, rising more than 6.5% to $32.16.
'Urgent public health priority'
The development of COVID-19 vaccine is "is an urgent public health priority," Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, said Monday in a statement regarding the Phase 1 trial in Seattle.
That trial, which Fauci said was launched in "record speed," will test the vaccine on 45 males and non-pregnant females ages 18 to 55, according to the NIH's website.
The number of coronavirus cases outside of mainland China is now greater than the number there, where the disease was discovered in late December.
There are more than 81,000 confirmed cases in China, nearly 28,000 in Italy and more than 16,000 in Iran. The U.S. has more than 5,200.
Although expectations are high, doctors have sought to keep expectations low for how quickly a vaccine can get to market. The process of developing, testing and reviewing potential vaccines is complex and lengthy, taking months or even years, global health experts say.
— CNBC's Berkeley Lovelace Jr. contributed to this report.


