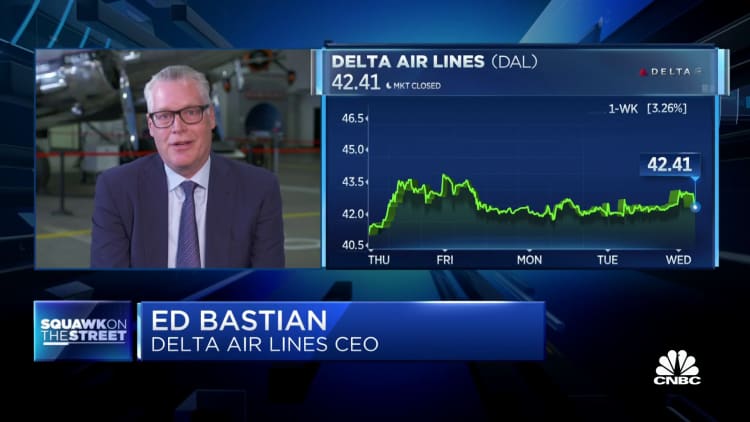
The Pfizer vaccine rollout continues for the second day in the U.K., but a health regulator has warned that people with a history of severe allergic reactions should skip the shot for now. Members of the U.S. Food and Drug Administration are set to meet and discuss the drug on Thursday. Briefing documents circulated by FDA staff on Tuesday ahead of the meeting confirmed the safety and efficacy of the Pfizer-BioNTech vaccine and said the drug offers some protection after the first dose.
Here are some of the biggest developments Wednesday:
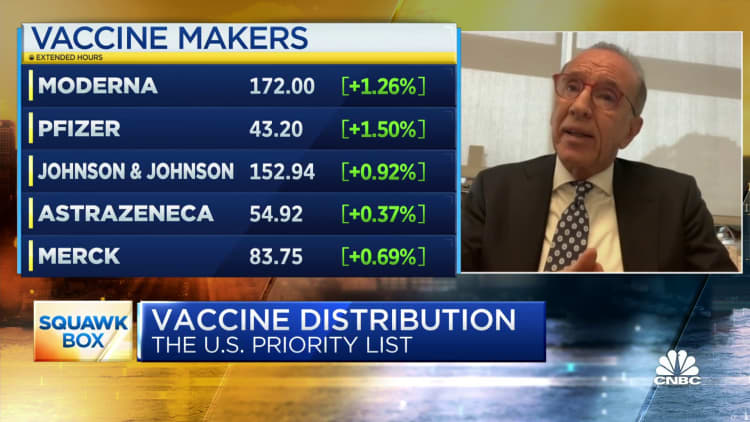
The following data was compiled by Johns Hopkins University:
- Global cases: More than 68.86 million
- Global deaths: At least 1.56 million
- U.S. cases: More than 15.38 million
- U.S. deaths: At least 289,373